Zygomatic Implants are the type of dental implant where the implant support is taken from the zygomatic bone and part of the alveolar bone
What are Zygomatic Implants?
Zygomatic Implants are the type of dental implant where the implant support is taken from the zygomatic bone and part of the alveolar bone of the maxilla. It is a graft-less technique and is done in patients who had bone graft failure in the past, atrophied maxilla, medically compromised patients and cannot tolerate extensive surgical procedures.
The advantages of choosing zygomatic implants are due to less involvement of surgical sites for implant placement, fewer surgical procedures and a shortened time frame between surgery and placement of the final prosthesis.
History of Zygomatic Implants
Branemark and colleagues 1988 introduced zygomatic implants to provide implant-supported prosthesis for patients with severe maxillary atrophy and maxillary defect from trauma and resections. After some decades of clinical studies, various companies introduced different variations of zygomatic implants. The companies are,
- Nobel Biocare
- Neodent
- Noris Medical
- Southern implants
- Implant Swiss
Noble Biocare implants are 30 to 52.5 mm in length. While Branemark zygomatic implants have a 45-degree abutment head and a Ti (Titanium) unite surface. Ti unit surface has a roughened and thick TiO2 layer which is highly porous.
Biomechanics of zygomatic implants
The stability and success of zygomatic implants are due to Quad anchoring of the implant to the maxilla and zygoma region.
Indications of Zygomatic Implants
- For Implant-supported rehabilitations of completely Edentulous patients with significant pneumatization and severe posterior alveolar resection.
- For Prosthetic rehabilitation for extensive defects of the maxilla due to trauma or neoplastic and congenital conditions.
- For Completely edentulous patients in Zone 2 and Zone 3
- Patient history of bone graft failure.
- Patient are unable to undergo bone grafts due to their medically compromised conditions.
Contraindications of zygomatic implants
Absolute contraindications are acute maxillary sinusitis and maxilla under zygoma pathological conditions uncontrolled/ malignant systemic diseases. Relative contraindications include chronic infectious sinusitis, prolonged use of bisphosphonate drugs, and smoking greater than 22 cigarettes/ day.
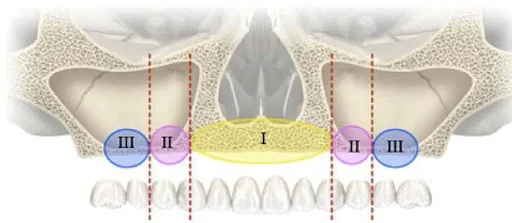
Bedrossian classification for maxillary bone availability for dental implants
- Zone I premaxilla
- Zone II pre-molar area
- Zone III molar area
The bone level assessment in the above zones is associated with clean Asian through cone beam computed tomography CBCT. The Placement of zygoma implants when there is less than 2-3 mm in Zone 2 and Zone 3 is suggested by Bedrossian.
If the edentulous patient has inadequate bone in all three zones, then the placement of four zygomatic implants are placed. This is also known as quad zygomatic implants.
ZAGA (ZYGOMA ANATOMY GUIDED APPROACH SYSTEM)
ZAGA concept was described as a refinement of the extrasinus technique for placement of zygomatic implants. According to this concept, the placement of the implant is guided by anatomical and Prosthetic requirements. It helps in understanding the possible anatomic variation between patients and even in different sites of the same patient. It provides zygomatic implants to specified anatomy. The implant may be Intrasinus or Extrasinus or in Multiple intermediary positions using the maxillary wall as an additional source of anchorage. The main aim of ZAGA is to improve the primary stability of prosthetically positioned zygomatic implants.
 |
Zygoma anatomic guided approach (ZAGA) Types
(A) ZAGA 0; (B) ZAGA 1; (C) ZAGA 2; (D) ZAGA 3; (E) ZAGA 4 |
A proposed classification for zygomatic implant based on the zygoma anatomy-guided approach (ZAGA)
|
ZAGA
TYPE
|
ANTERIOR
MAXILLA
|
IMPLANT
HEAD LOCATION
|
IMPLANT
BODY PATH
|
IMPLANT
CONTACT WITH BONE
|
|
ZAGA 0
|
Very Flat
|
Alveolar Crest
|
Intrasinus
|
Alveolar crest, Zygomatic Bone, Lateral sinus wall
(partially)
|
|
ZAGA 1
|
Slight Concave
|
Alveolar Crest
|
Intrasinus
|
Alveolar crest, Zygomatic Bone, Lateral sinus wall
|
|
ZAGA 2
|
Concave
|
Alveolar Crest
|
Mostly extra-sinus
|
Alveolar crest, Zygomatic Bone, Lateral sinus wall
|
|
ZAGA 3
|
Very Concave
|
Alveolar Crest
|
Mostly extra-sinus
|
Alveolar crest, Zygomatic Bone
|
|
ZAGA 4
|
Extremely vertical and horizontal atrophy
|
Buccal to alveolar crest
|
Extrasinus, extramaxillary
|
Zygomatic Bone, Lateral sinus wall (partially)
|
Surgical Approaches for Zygomatic Implants
It depends on the hands of the operators, and patient factors.
- Anatomically driven - ZAGA concept
- Prosthetically driven- RAZIR (Restoratively Aimed Zygomatic Implant Routine)
Prosthetic reconstruction of the edentulous maxilla with zygoma implants done in
- Two-stage protocol
- Immediate loading protocol
Surgical Techniques for Zygomatic Implants
It is done under
Local anesthesia, i.v sedation, or General anesthesia. It is decided by the surgeon based on the patient's medical status, desired level of comfort and length of the procedure.
Local anesthesia via Local infiltration & blocks including posterior superior alveolar nerve block ( PSANB ), Greater palatine nerve block,
Inferior alveolar nerve block (IANB), Bilateral transcutaneous infiltration of the temporal area over the body of zygoma.
Complications of zygomatic implants
Sinusitis and failure of osseointegration of zygomatic implants and there is a chance of temporary neurosensory disturbances in the malar region of proximity zygomatic nerve (CNV2) will resolve in 3-8 weeks. The late complications occur 3 months after surgery and it is associated with the surgical techniques.
QUAD ZYGOMA PROTOCOL
In this protocol, 4 Implants are used. Out of which 2 implants are placed bilaterally with appropriate anterior-posterior spread and inclination. Quad zygoma protocol is the 1st choice of treatment in severe maxillary atrophy and an alternate treatment for bone graft technique and implant failure scenarios.
Contraindications of QUAD ZYGOMA PROTOCOL
The prolonged untreated periodontal diseases, poor oral hygiene, patient with restricted mouth opening, Acute or chronic inflammation in implant sites, malar bone abnormalities and Acute or chronic sinusitis with obstruction in the osteomeatal complex.














COMMENTS